As a novel biopharmaceutical technology, mRNA medicines have attracted much attention due to their potential in the treatment and prevention of diseases. Tangential flow filtration (TFF) technology, characterized by high efficiency and high recovery, plays a great role in mRNA medicines preparation.
What is mRNA?
mRNA is a type of ribonucleic acid in a cell that carries genetic information and directs the cell to make specific proteins. In mRNA medicines, synthetic mRNAs are designed to encode therapeutic proteins that can be used to treat or prevent disease.
Principles of mRNA medicine
- Coding design: scientists design and synthesize a specific mRNA sequence that encodes a therapeutic protein.
- Delivery into the cell: To ensure that the mRNA medicine effectively enters the patient's cells, an effective delivery system such as lipid nanoparticles (LNP) is required, which encapsulate and protect the mRNA, preventing it from being enzymatically degraded or failing to penetrate the cellular membrane before it reaches the site of action.
- Protein synthesis: once the mRNA enters the cell, it is recognized by the cell's protein synthesis machinery and directs the cell to synthesize the required therapeutic proteins.
Application of TFF to mRNA medicine preparation
- Steps of mRNA production process

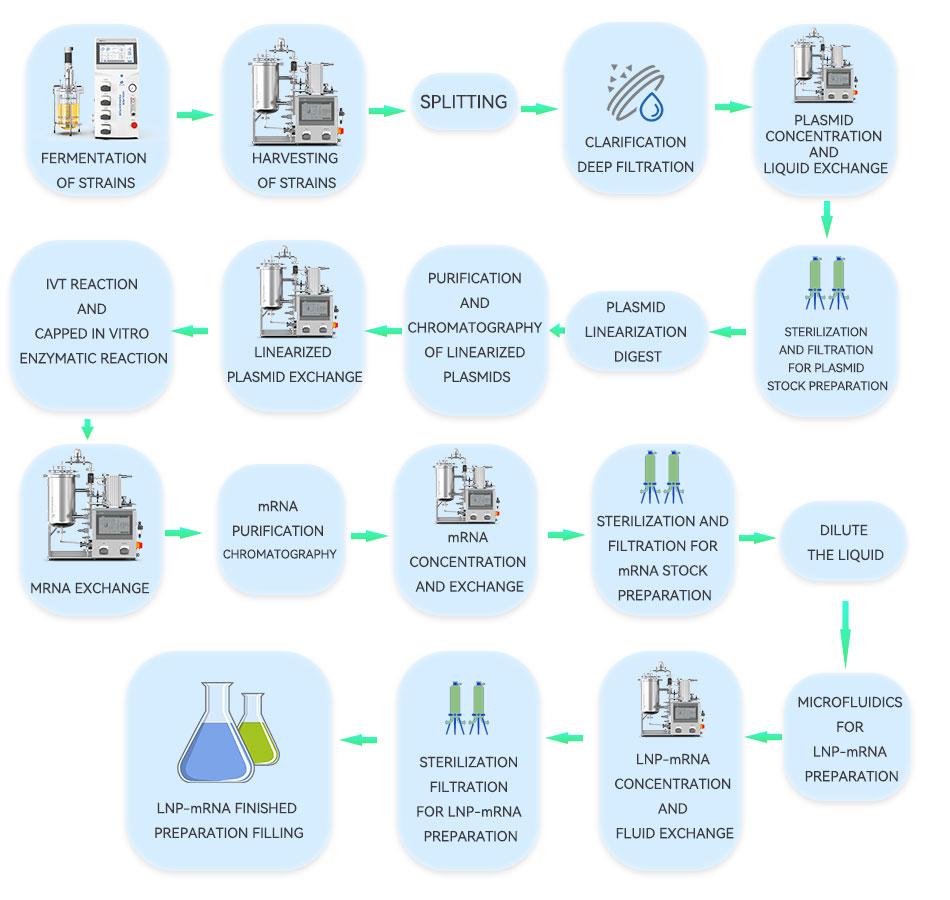
The steps of mRNA production process are basically divided into the above three steps, the plasmid DNA stock preparation is based on the sequence design of the transcription template, and the preparation method usually adopts plasmid DNA amplification, or PCR amplification can also be used. Take DNA amplification as an example, it is usually amplified by fermentation of engineering bacteria E.coli. Strain recovery is carried out first, and the production of plasmid DNA goes through the steps of fermentation and culture, harvesting and clarification, refinement and purification, linearization, and dispensing and storage. mRNA stock preparation mainly includes the in vitro transcription (IVT) of linearized plasmid DNA into mRNA, isolation and purification after chemical modification, and dispensing of purified mRNA stock for freezing and storing. mRNA preparation mainly includes the processes of mRNA- LNP encapsulation preparation, purification, decontamination and filtration, and aseptic filling. We will focus on which steps need to use the TFF process in this paper, how to choose membrane packages and hollow fibers, and what specifications are used.
- Plasmid DNA stock preparation
The main process of upstream plasmid amplification is through E. coli fermentation, and after the culture is finished, the bacterium needs to be collected. Laboratory centrifuges are limited by their operation to meet commercial scale production, and industrial continuous flow centrifuges have high shear. Hollow fibers, on the other hand, have open channels and are capable of handling high solids, high viscosity, shear-sensitive samples, and have good scale-up capabilities. The collection was performed using a 750kD/100nm hollow fiber membrane column to remove cellular debris and media components. Next, bacterial lysis was performed using high pressure homogenizer crushing, ultrasonic lysis, and alkaline lysis, and clarification was performed by deep filtration. To facilitate chromatographic purification, TFF was used for concentration and liquid exchange before chromatographic up-sampling to greatly reduce the sample volume and to remove some impurities such as RNA, HCP and HCD.
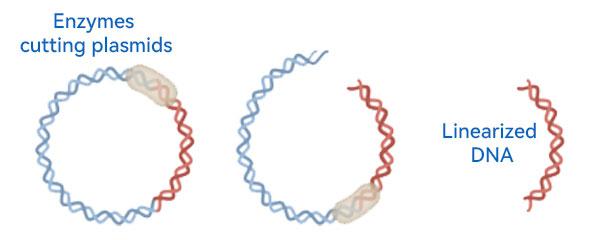
According to the molecular weight of the plasmid DNA, 30kD/100kD/300kD is used, and the TMP is less than 0.5 bar, and then it will be refined and purified by chromatography. After chromatography, the purity of plasmid DNA is already very high, according to the need of linearization step, we can first carry out ultrafiltration to concentrate and exchange the liquid, according to the molecular weight size of plasmid DNA, 30kD/100kD/300kD, and then use restriction endonuclease to complete the linearization of plasmid DNA. After linearization, the microbial load is controlled by sterilizing filtration.
As plasmid DNA is an intracellular product, and the production of mRNA of specific length and sequence requires highly purified and linearized plasmid raw materials, the entire downstream purification process process route is more complex, the yield is not high (30%~40%), which results in the industry's plasmid production cost is higher, how to optimize and improve the downstream purification process, has become the current plasmid DNA preparation link in the urgent need to solve the problem.
According to the molecular weight of the plasmid DNA, 30kD/100kD/300kD is used, and the TMP is less than 0.5 bar, and then it will be refined and purified by chromatography. After chromatography, the purity of plasmid DNA is already very high, according to the need of linearization step, we can first carry out ultrafiltration to concentrate and exchange the liquid, according to the molecular weight size of plasmid DNA, 30kD/100kD/300kD, and then use restriction endonuclease to complete the linearization of plasmid DNA. After linearization, the microbial load is controlled by sterilizing filtration.
As plasmid DNA is an intracellular product, and the production of mRNA of specific length and sequence requires highly purified and linearized plasmid raw materials, the entire downstream purification process process route is more complex, the yield is not high (30%~40%), which results in the industry's plasmid production cost is higher, how to optimize and improve the downstream purification process, has become the current plasmid DNA preparation link in the urgent need to solve the problem.
- mRNA stock solution preparation
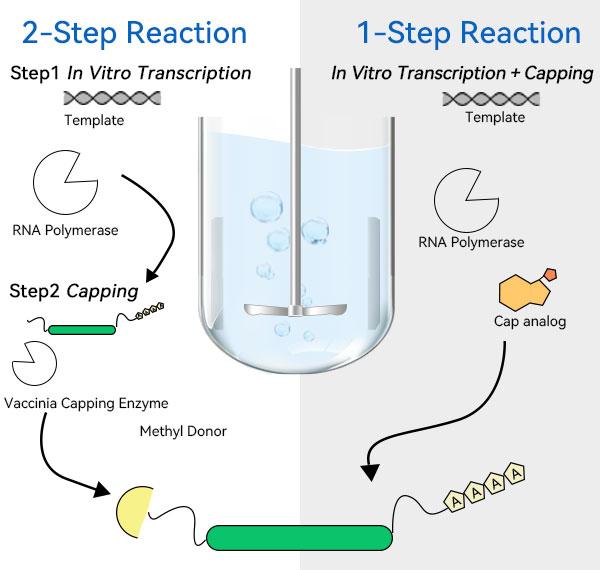
IVT and modification is the key process for mRNA stock preparation, which involves multiple steps, including transcription, 5' end capping, 3' end Poly(A) tailing, dephosphorylation, etc. After transcription and modification, RNA polymerase can be removed by using a membrane packet of 30kD/100kD/300kD and a hollow fiber membrane column, DNA fragments, NTPs, capsidases, dsRNA, inhibitors and other impurities, and replace the buffer. then use affinity chromatography, size exclusion chromatography, ion-pair reversed-phase chromatography, ion-exchange chromatography, and other chromatographic processes for refinement and purification, after the aforementioned ultrafiltration, chromatography, and other purification steps, the mRNA has already reached a considerable purity, and then use the 30 kD/100 kD/300 kD membrane package or hollow fiber membrane column to adjust the purity. Then use 30kD/100kD/300kD membrane packages or hollow fiber membrane columns to adjust the concentration of mRNA in the stock solution and replace the buffer, and then use sterilizing filtration to control the microbial load for temporary storage and encapsulation.
- mRNA preparation
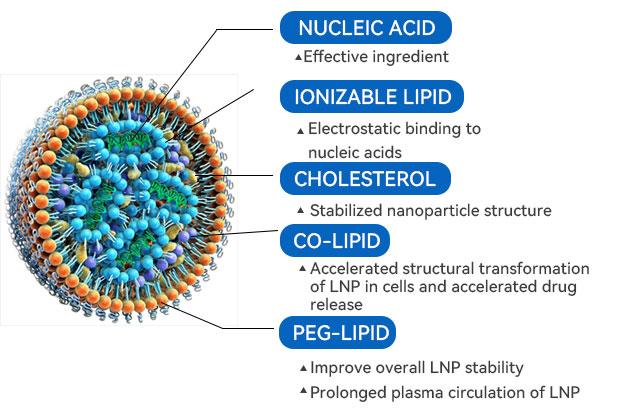
The mRNA-LNP encapsulation preparation is the key process for the preparation of mRNA preparation, except for the formulation of lipid components, it is the control of the process, i.e., how to control the process of contact and interaction between mRNA and lipid components by microfluidics in order to form a stable, homogeneous, and high yielding mRNA-LNP complex. mRNA-LNP solution was concentrated and exchanged by 100kD/300kD TFF technique for concentration exchange, the mRNA-LNP complexes were retained, and ethanol and preparations were washed and filtered. Final sterilization filtration was performed using a redundant setup of 0.22 μm sterilization grade filters to intercept microbial contaminants such as bacteria. Finally, aseptic filling was performed.
 While mRNA technology has made breakthroughs in the field of vaccines for infectious diseases, the application of mRNA technology in other fields is also unfolding. We expect that with the advancement of science and technology, mRNA technology will be more widely used for the benefit of patients!
While mRNA technology has made breakthroughs in the field of vaccines for infectious diseases, the application of mRNA technology in other fields is also unfolding. We expect that with the advancement of science and technology, mRNA technology will be more widely used for the benefit of patients!
Here is the Holves brand website, https://www.bjholves.com/. Providing different types of industry information, technical knowledge, and solutions, we have developed and produced several new laboratory fermenter, bioreactor, tangential flow filtration system and other equipment to meet your needs from experimental to industrial production.


Here is the Holves brand website, https://www.bjholves.com/. Providing different types of industry information, technical knowledge, and solutions, we have developed and produced several new laboratory fermenter, bioreactor, tangential flow filtration system and other equipment to meet your needs from experimental to industrial production.
