At the end of 2023, the journal Science named the GLP-1 class of drugs the top 10 scientific breakthroughs of the year (Science's 2023 Breakthrough of the Year), while Nature named GLP-1 research pioneer Svetlana Mojsov as one of the top 10 people of the year of the year. At the business level, GLP-1 drugs also deserved to be the star of the year. In 2023, Novo Nordisk's ace product, simethicone, sold $21.157 billion and ranked second in the world, just one step away from being the king of the world's drugs.
In the treatment of type 2 diabetes mellitus, GLP-1 analogs have become a good helper in diabetes treatment due to their excellent hypoglycemic effect. However, the rapid degradation property of GLP-1 limits its wide use as a drug to some extent. To overcome this challenge, tangential flow filtration (TFF) technology plays a crucial role in the downstream process of GLP-1 drugs, which effectively extends the stability and half-life of GLP-1 analogs through a sophisticated filtration process, thus providing patients with safer and more effective treatment options.
In the treatment of type 2 diabetes mellitus, GLP-1 analogs have become a good helper in diabetes treatment due to their excellent hypoglycemic effect. However, the rapid degradation property of GLP-1 limits its wide use as a drug to some extent. To overcome this challenge, tangential flow filtration (TFF) technology plays a crucial role in the downstream process of GLP-1 drugs, which effectively extends the stability and half-life of GLP-1 analogs through a sophisticated filtration process, thus providing patients with safer and more effective treatment options.
ABOUT GLP-1
GLP-1, known as glucagon-like peptide-1, is a hormone secreted by intestinal cells that is rapidly released after a meal to stimulate the secretion of insulin from pancreatic β-cells, thus lowering blood glucose.GLP-1 does more than just that, it slows down the rate of gastric emptying, increases satiety, and reduces food intake, thus helping to control body weight. However, natural GLP-1 is not stable when it enters the body and is easily and quickly broken down, so people have been developing improvements to produce a long-lasting GLP-1 drug.
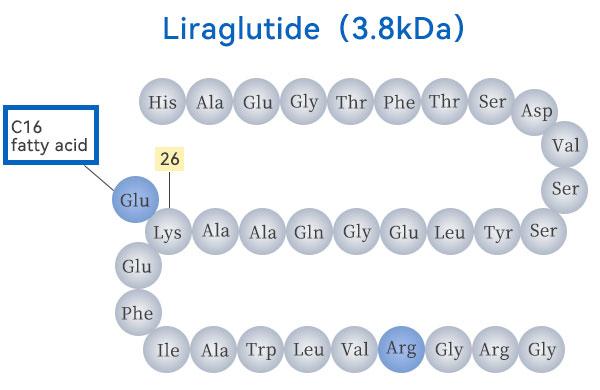
ABOUT GLP-1 GRUGS
The production and preparation of GLP-1 drugs, which are peptide drugs consisting of multiple amino acids linked by peptide chains, is a complex process. They are divided into two categories: bio-fermentation synthesis and chemical synthesis.
Bio-fermentation synthesis expresses inclusion bodies through E. coli or yeast fermentation, followed by a series of steps such as harvesting, breaking, denaturing, and clarification.Chemical synthesis adds amino acids to the growing peptide chain one by one through solid or liquid phase synthesis techniques.Either method ultimately requires process steps such as chromatography and UF to purify and adjust the concentration of the drug.
Bio-fermentation synthesis expresses inclusion bodies through E. coli or yeast fermentation, followed by a series of steps such as harvesting, breaking, denaturing, and clarification.Chemical synthesis adds amino acids to the growing peptide chain one by one through solid or liquid phase synthesis techniques.Either method ultimately requires process steps such as chromatography and UF to purify and adjust the concentration of the drug.
APPLICATION OF TFF IN GLP-1 GRUG PREPARATION
- Selection of membrane material
In the chemical synthesis process of GLP-1 and subsequent chemical modification, organic solvents are often required to dissolve the reactants and products, accelerate the reaction process and facilitate the separation and purification of the products. Common organic solvents include methanol, ethanol, acetone and dimethyl sulfoxide. Among these solvents, cellulose shows better compatibility than polyethersulfone, and its lower protein adsorption makes regenerated cellulose (RC) the preferred filtration material for the GLP-1 ultrafiltration/defiltration (UF/DF) process.
- Selection of membrane pore size
The molecular weight of the GLP-1 target peptide is 3.4 KD, and in ultrafiltration, the criteria for selecting the MWCO is usually 1/3 to 1/5 of the target MWCO. Based on this principle, we recommend the use of membranes with a MWCO of 1-2 KD for the concentration and buffer exchange to ensure the integrity and activity of the peptide.
- Downstream processes
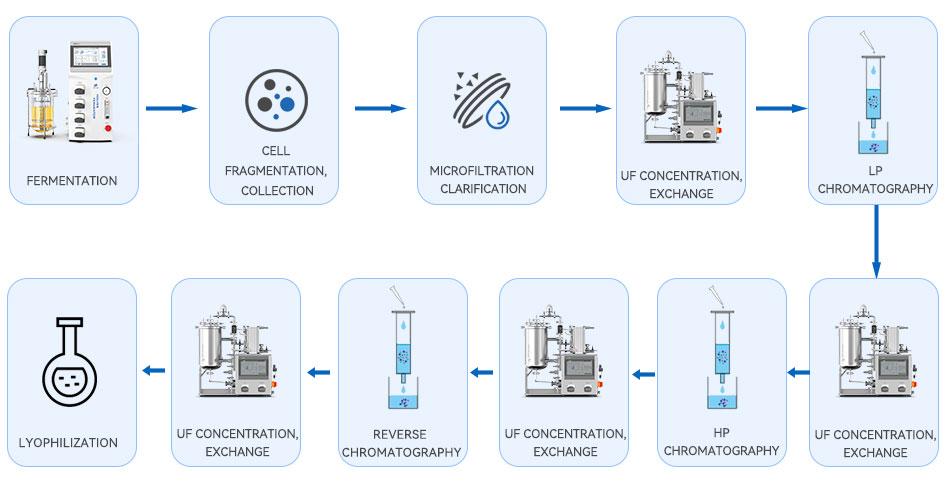
From the process flow, it can be seen that UF/DF is involved in many of the downstream processes of GLP-1, which not only plays the functions of concentration, buffer replacement, etc., but also effectively reduces the pressure of the subsequent chromatography process and improves the overall production efficiency and product quality.
Here is the Holves brand website, https://www.bjholves.com/. Providing different types of industry information, technical knowledge, and solutions, we have developed and produced several new laboratory fermenter, bioreactor, tangential flow filtration system and other equipment to meet your needs from experimental to industrial production.
Here is the Holves brand website, https://www.bjholves.com/. Providing different types of industry information, technical knowledge, and solutions, we have developed and produced several new laboratory fermenter, bioreactor, tangential flow filtration system and other equipment to meet your needs from experimental to industrial production.








