Since the first therapeutic antibody entered the clinic in 1986, the therapeutic antibody drugs monoclonal antibody, biclonal antibody, and ADC have been rapidly developed. So far, the FDA has approved nearly one hundred therapeutic antibody drugs, of which those involving the immune system and tumor therapy account for 68%. Antibody drugs have become an important part of modern biomedicine.
Currently, among the products of monoclonal antibody preparations, the number of high-concentration preparation products approved by FDA after 2015 is three times the total number approved before 2015. This shows the trend of high-concentration formulations. The focus of this article is to chat about the challenges and considerations of the TFF process in the development of highly concentrated antibodies.
Although the advantages of high-concentration preparations are obvious, high-concentration antibody preparations are more susceptible to Self- association and aggregation due to their more complex intermolecular structure.
In order to achieve the high protein concentrations required for subcutaneous administration, many production process challenges are often encountered. From an operational dimension, high protein concentrations result in high viscosities, which can lead to increased pressure during the ultrafiltration concentration process. It can also lead to a gradual decrease in the flux of the ultrafiltration concentration process to a very low level, which can lead to longer processing times.
The process of concentrating highly concentrated preparations brings about several changes in the material and the TFF system:
1、the rise of concentration and viscosity
Elevated concentration will make the fluidity of the material worse. Higher protein concentration has a higher risk of aggregation, because the closer the component molecules, the stronger the intermolecular interaction, the higher the probability of self-association.
2、Size exclusion
Size exclusion is mainly due to the fact that proteins with high concentration in the upstream of the membrane occupy a larger volume, resulting in less space for other solutes. Especially for large size molecules, it will lead to a difference in the concentration of some solute molecules in the upstream and downstream of the membrane. The osmotic pressure increases as the osmotic concentration changes upstream and downstream of the membrane.
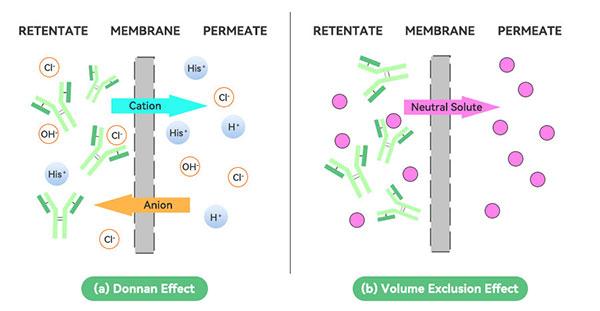
3、Donan effect
Charged products are retained by the ultrafiltration membrane, and the presence of charged groups makes the membrane retain different ions at different rates. When there are a variety of ions in solution, the charge size and ionic radius of different ions will affect the proportion of them through the membrane. The larger the charge and smaller the radius of the ions, the lower the proportion of them passing through the membrane, which leads to changes in the concentration and pH of the solution.
4、Pressure changes
In the ultrafiltration process, as the concentration continues, the protein concentration rises, especially in the late stage of concentration, the high viscosity of the material leads to a rise in system resistance and the pressure drop becomes larger. In the flow path of the membrane package, the pressure drop is more obvious. Finally, the inlet pressure of the membrane package rises rapidly, exceeding the maximum inlet pressure limit, resulting in concentration not proceeding.
 We can improve the control process in several ways:
We can improve the control process in several ways:
1、Choose the right buffer
The type, pH, and concentration of the buffer affects the protein surface charge distribution, type, and amount, and further affects protein conformation and colloidal stability by influencing electrostatic interactions. To achieve the target formulation composition, the DF buffer or ultrafiltration dilution buffer can be adjusted to address excipient concentration shifts. Alternatively, the observed Dornan effect can be reduced by using a buffer of high ionic strength during the UF step.
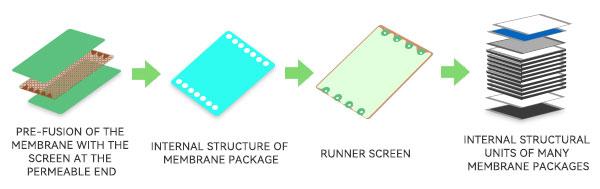
2、Choose the right film package screens
Different brands of flat film packages have a variety of screen options. Highly concentrated and viscous protein preparations can choose the suspended turbulent flow screen. The optimized screen structure can reduce the pressure loss, slow down the phenomenon of high inlet pressure; at the same time effectively generate turbulence, improve the permeability effect, slow down the concentration polarization that is more likely to occur in high concentrations, improve the flux.
 3、Choice of hollow fiber columns
3、Choice of hollow fiber columns
Hollow fiber columns are an excellent filtration material for the preparation of highly concentrated antibody preparations compared to flat membrane packs. Because the inner lumen of the fiber exhibits non-turbulent hydrodynamics, a significantly lower pressure drop can be obtained. Concentration of monoclonal antibodies by 0.5 and 1.0 mm I.D. fibers, we found that there is a performance difference between the two, with 1.0 mm I.D. fibers obtaining a lower pressure drop and higher throughput, resulting in shorter processing times.
4、Using Single-pass TFF
SPTFF technology is an application of tangential flow filtration technology, which differs from normal TFF in that the fluid passes through the ultrafiltration membrane in a single pass and there is no recirculation process.
 SPTFF technology also utilizes a very low flow rate to increase the conversion rate to achieve the target protein concentration.SPTFF technology also simplifies the ultrafiltration system configuration and simplifies the operation. Due to the lower inlet flow rate, the configuration of pumps,vessels, piping, etc. is reduced, smaller system recirculation volume and retention volume, increasing the final concentration that can be achieved and improving the product yield; the inlet flow rate is reduced, and the high concentration will not cause high inlet pressure alarm.
SPTFF technology also utilizes a very low flow rate to increase the conversion rate to achieve the target protein concentration.SPTFF technology also simplifies the ultrafiltration system configuration and simplifies the operation. Due to the lower inlet flow rate, the configuration of pumps,vessels, piping, etc. is reduced, smaller system recirculation volume and retention volume, increasing the final concentration that can be achieved and improving the product yield; the inlet flow rate is reduced, and the high concentration will not cause high inlet pressure alarm.
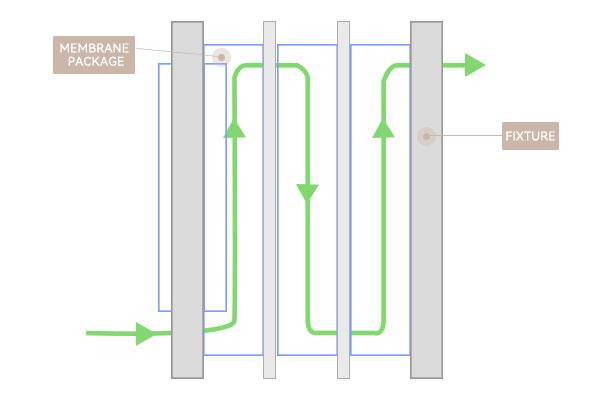
In the SPTFF technology, the material only passes through the pump once, reducing the shear force, reducing the impact on the amount of protein under high concentration conditions, greatly improving the stability of the protein and reducing the generation of polymers. More conveniently, SPTFF can be realized using existing membrane packages and fixtures.
In recent years, highly concentrated antibody formulations have gained attention for their therapeutic potential and ease of administration to patients. However, the development of these products has been challenging at the manufacturing, stability, analytical and regulatory levels. These challenges can only be addressed through robust manufacturing processes and formulation development, ultimately leading to commercially available highly concentrated antibody products.
Here is the Holves brand website, https://www.bjholves.com/. Providing different types of industry information, technical knowledge, and solutions, we have developed and produced several new laboratory fermenter, bioreactor, tangential flow filtration system and other equipment to meet your needs from experimental to industrial production.
About HCPF
In antibody experimentation and production, high concentration protein formulations (HCPF) are the goal pursued by many pharmaceutical companies.HCPF usually refers to formulations with protein concentration higher than 50mg/ml. High concentration preparation can not only reduce the pain of injection for patients, but also reduce costs and save medical resources.Currently, among the products of monoclonal antibody preparations, the number of high-concentration preparation products approved by FDA after 2015 is three times the total number approved before 2015. This shows the trend of high-concentration formulations. The focus of this article is to chat about the challenges and considerations of the TFF process in the development of highly concentrated antibodies.
TFF & HCPF
Although the advantages of high-concentration preparations are obvious, high-concentration antibody preparations are more susceptible to Self- association and aggregation due to their more complex intermolecular structure.In order to achieve the high protein concentrations required for subcutaneous administration, many production process challenges are often encountered. From an operational dimension, high protein concentrations result in high viscosities, which can lead to increased pressure during the ultrafiltration concentration process. It can also lead to a gradual decrease in the flux of the ultrafiltration concentration process to a very low level, which can lead to longer processing times.
The process of concentrating highly concentrated preparations brings about several changes in the material and the TFF system:
1、the rise of concentration and viscosity
Elevated concentration will make the fluidity of the material worse. Higher protein concentration has a higher risk of aggregation, because the closer the component molecules, the stronger the intermolecular interaction, the higher the probability of self-association.
2、Size exclusion
Size exclusion is mainly due to the fact that proteins with high concentration in the upstream of the membrane occupy a larger volume, resulting in less space for other solutes. Especially for large size molecules, it will lead to a difference in the concentration of some solute molecules in the upstream and downstream of the membrane. The osmotic pressure increases as the osmotic concentration changes upstream and downstream of the membrane.
3、Donan effect
Charged products are retained by the ultrafiltration membrane, and the presence of charged groups makes the membrane retain different ions at different rates. When there are a variety of ions in solution, the charge size and ionic radius of different ions will affect the proportion of them through the membrane. The larger the charge and smaller the radius of the ions, the lower the proportion of them passing through the membrane, which leads to changes in the concentration and pH of the solution.

In the ultrafiltration process, as the concentration continues, the protein concentration rises, especially in the late stage of concentration, the high viscosity of the material leads to a rise in system resistance and the pressure drop becomes larger. In the flow path of the membrane package, the pressure drop is more obvious. Finally, the inlet pressure of the membrane package rises rapidly, exceeding the maximum inlet pressure limit, resulting in concentration not proceeding.
How to improve the process

1、Choose the right buffer
The type, pH, and concentration of the buffer affects the protein surface charge distribution, type, and amount, and further affects protein conformation and colloidal stability by influencing electrostatic interactions. To achieve the target formulation composition, the DF buffer or ultrafiltration dilution buffer can be adjusted to address excipient concentration shifts. Alternatively, the observed Dornan effect can be reduced by using a buffer of high ionic strength during the UF step.
2、Choose the right film package screens
Different brands of flat film packages have a variety of screen options. Highly concentrated and viscous protein preparations can choose the suspended turbulent flow screen. The optimized screen structure can reduce the pressure loss, slow down the phenomenon of high inlet pressure; at the same time effectively generate turbulence, improve the permeability effect, slow down the concentration polarization that is more likely to occur in high concentrations, improve the flux.

Hollow fiber columns are an excellent filtration material for the preparation of highly concentrated antibody preparations compared to flat membrane packs. Because the inner lumen of the fiber exhibits non-turbulent hydrodynamics, a significantly lower pressure drop can be obtained. Concentration of monoclonal antibodies by 0.5 and 1.0 mm I.D. fibers, we found that there is a performance difference between the two, with 1.0 mm I.D. fibers obtaining a lower pressure drop and higher throughput, resulting in shorter processing times.
4、Using Single-pass TFF
SPTFF technology is an application of tangential flow filtration technology, which differs from normal TFF in that the fluid passes through the ultrafiltration membrane in a single pass and there is no recirculation process.

In the SPTFF technology, the material only passes through the pump once, reducing the shear force, reducing the impact on the amount of protein under high concentration conditions, greatly improving the stability of the protein and reducing the generation of polymers. More conveniently, SPTFF can be realized using existing membrane packages and fixtures.
In recent years, highly concentrated antibody formulations have gained attention for their therapeutic potential and ease of administration to patients. However, the development of these products has been challenging at the manufacturing, stability, analytical and regulatory levels. These challenges can only be addressed through robust manufacturing processes and formulation development, ultimately leading to commercially available highly concentrated antibody products.
Here is the Holves brand website, https://www.bjholves.com/. Providing different types of industry information, technical knowledge, and solutions, we have developed and produced several new laboratory fermenter, bioreactor, tangential flow filtration system and other equipment to meet your needs from experimental to industrial production.








